
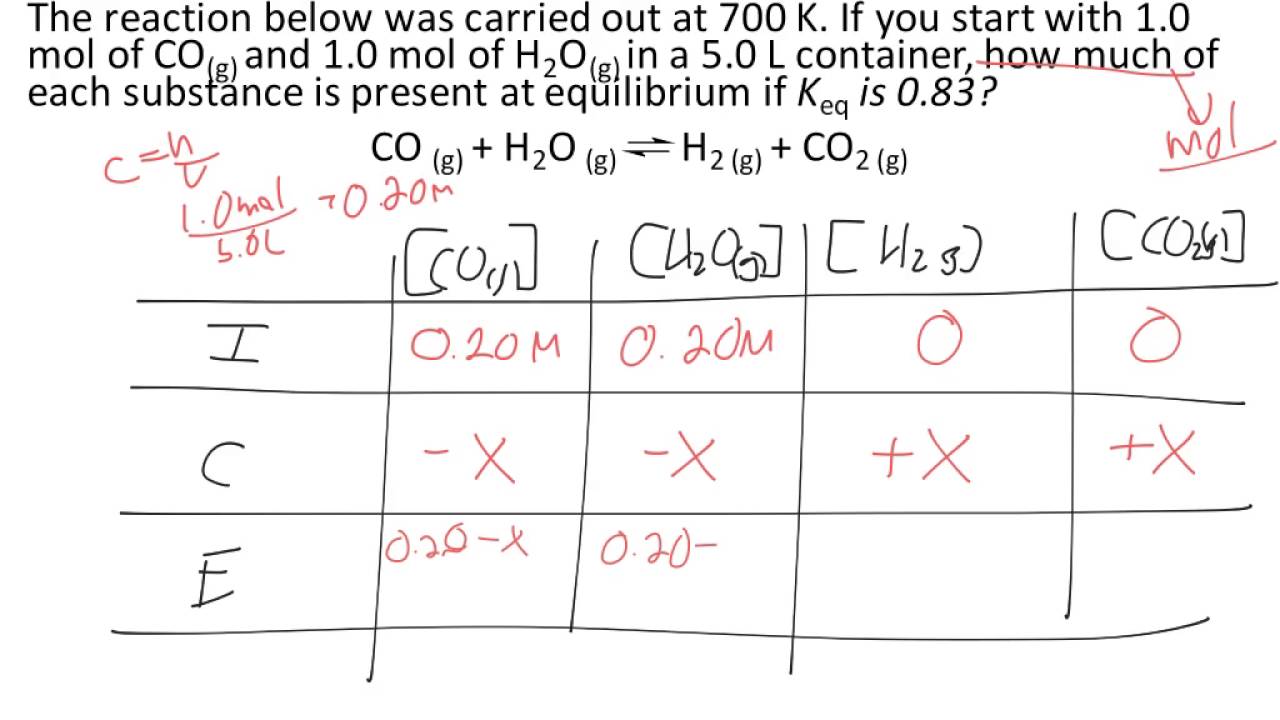
This implies starting with the initial concentrations, determining the change, and using K to find the equilibrium concentrations.Įxample "Start, change, equilibrium" problem:Ĭ 2H 6(g) + Cl 2(g) C 2H 5Cl(s) + HCl(g) To find the concentrations which characterize an equilibrium, it is best to proceed through the "start, change, equilibrium" process. If Q is equal to K than the system is already at equilibrium so it will not shift in either direction. If Q is less than K, the system will shift to the right. If Q is greater than K, the system will shift to the left. We compare Q and K to determine which direction the reaction will proceed to obtain equilibrium. The reaction quotient, Q, is an expression which deals with initial values instead of the equilibrium value that K deals with.
R is the gas law constant (see the gas laws page) K p, the equilibrium constant in terms of pressure, is related to K by the equation:ĭelta n is the sum of the coefficients of the gaseous products minusthe sum of the coefficients of the gaseous reactants. It is critical to remember that the only thing that changes K is changing temperature.įor reactions in the gas phase, equilibrium positions can also be expressed in terms of pressure.

A small K value implies there are more reactants than products and the reaction lies to the left. A large value of K implies that there are more products than reactants and that the equilibrium lies to the right. are the coefficients of the balanced reactionįor every reaction at a specific temperature, there is only one valuefor K. are the molar concentrations of A, B, etc. It is important to remember that only species in either the gas or aqueous phases are included in this expression because the concentrations for liquids and solids cannot change. It is possible to write an equilibrium expression for a reaction.This can be expressed by concentrations of the products divided by theconcentration of the reactants with the coefficients of each equationacting as exponents. This is why equilibrium is also referred to as"steady state". A reverse reaction is when the written reaction goes from right to left instead of the forward reaction which proceeds from left to right. A reaction may look "finished" when equilibrium is reached, but actually the forward and reverse reactions continue to happen at the same rate. Some may appear to be completely products, however, all reactions have some reactants present.

Different reactions have different equilibriums.

Equilibrium occurs when there is a constant ratio between the concentration of the reactants and the products. However, when a chemical reaction is carried out in a closed vessel, the system achieves equilibrium. The procedure was successfully tested on vapor-liquid equilibrium (VLE) and vapor-liquid-liquid equilibrium (VLLE) of reaction systems.In stoichiometry calculations, we assume that reactions run to completion. Stability analysis is used to introduce additional phases sequentially so as to obtain the final multiphase solution. The whole solution procedure employs a nested loop with Newton iteration in the inner loop and non-ideality updated in the outer loop, thus giving an overall linear convergence rate. This initialization is the unconstrained minimization of a convex function and it is bound to converge. Lagrange multipliers and phase amounts are the independent variables, whose initialization is performed by solving a subset of the working equations. We selected the method of Lagrange multipliers to minimize the Gibbs energy of the system, under material balance constraints. The purpose of this work is to develop a general, reliable and efficient algorithm, which is able to deal with multiple reactions in multiphase systems.


 0 kommentar(er)
0 kommentar(er)
