
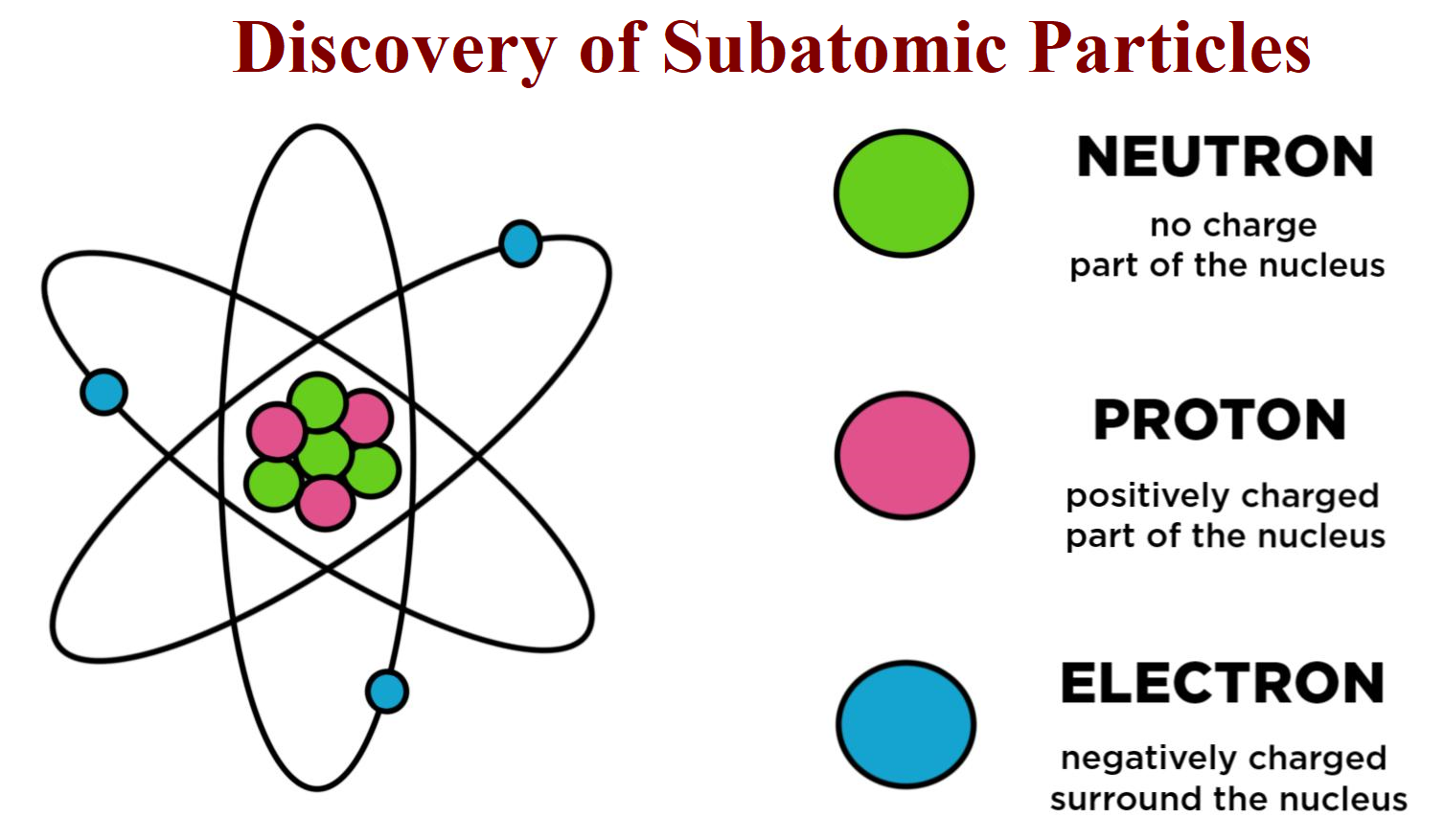
Though known to have serious defects, the Bohr model still supplies the standard graphic representation of the atom: a solid nucleus around which electrons orbit like tiny planets. The Bohr model also requires that the angular momentum (mass times velocity times distance from the orbital center) of the electron be limited to certain values (that is, be quantized) in order that the electron not fall into the nucleus. In this model, the hydrogen atom consists of an electron orbiting the nucleus (a single proton), much as Earth orbits the sun. In 1913, the Bohr model of the atom was introduced (named after Danish physicist Neils Bohr ). From their observations of the angles at which alpha particles were deflected, they deduced that atoms had relatively hard and small centers, thus proving the existence of the atomic nucleus and disproving the plum-pudding model. In 1909 –1911, English physicist Ernest Rutherford (1871 –1937) and his colleagues, German physicist Hans Wilhelm Geiger (1882 –1947) and New Zealand physicist Ernest Marsden (1888 –1970) did their famous scattering experiments involving alpha particles (two protons and two neutrons a helium-atom nucleus) shot through gold foil. Thomson thought that protons and electrons were randomly scattered throughout the atom, the so-called plum-pudding model. These were in fact, hydrogen nuclei (protons), but atomic structure was not understood at the time. Thomson reported detecting positively charged hydrogen atoms. ”) In 1906, the first clues to the nature of the proton were seen. (The word proton is Greek for the “first one. The proton was one of the earliest particles known. Both words convey mental pictures that are useful in some physical applications, but neither picture is sufficient: a photon is not a particle in the sense of a perfectly round, hard, self-contained sphere, nor is light a wave in the sense of being a smooth undulation in some medium.) Protons (The word wave is applied by physicists to describe some observable aspects of the behavior of light, while the particle terminology of the photon is applied to describe others. A photon (the name was coined by American chemist Gilbert Lewis in 1926) is one of these quanta, the smallest possible piece of energy in a light wave.

In 1905, German-American physicist Albert Einstein (1879 –1955) studied the photoelectric effect and proposed that radiation is quantized by its nature -that is, transfers energy in minimal packets termed quanta. In 1900, German physicist Max Planck (1858 – 1947) reported that light came in little packages of energy, which he called quanta. The first mediator particle to be discovered was the photon. The electron has a charge, e, of 1.6 ×10 -19 Coulombs (a unit of electrical charge named for French physicist, Charles-Augustin de Coulomb ). The charges of all particles are traditionally measured in terms of the size of the charge of the electron. Thomson gave it the name corpuscle, which was later changed to electron. The charge-to-mass ratio was found to be relatively large, and independent of the gas used in his experiments, which indicated to him that he had found a true particle. Thomson (1856 –1940), in 1897, measured the velocity and charge-to-mass ratio of these particles. Other scientists had deduced the existence of a negatively charged particle in what were called cathode rays (and which are now known to be beams of electrons). The first subatomic particle to be discovered was the electron. All forces, including gravity, are thought to be mediated by particle exchanges. That is, when two electrons collide, they do not simply bounce off of each other like two billiard balls: they exchange a photon (one of the mediator particles). These mediator particles enable the matter particles to interact with each other. The other elementary particles are mediators of the fundamental forces. The most famous baryons are protons and neutrons. Baryons and mesons are combinations of quarks and are considered subatomic particles. Examples of these particles include quarks (which make up protons and neutrons) and electrons. There are two types of elementary particles. However, the list of subatomic particles has now been expanded to include a large number of elementary particles and the particles they can be combined to make.

Early in the twentieth century, electrons, protons, and neutrons were thought to be the only subatomic particles these were also thought to be elementary (i.e., incapable of being broken down into yet smaller particles). Subatomic particles are particles that are smaller than an atom.


 0 kommentar(er)
0 kommentar(er)
